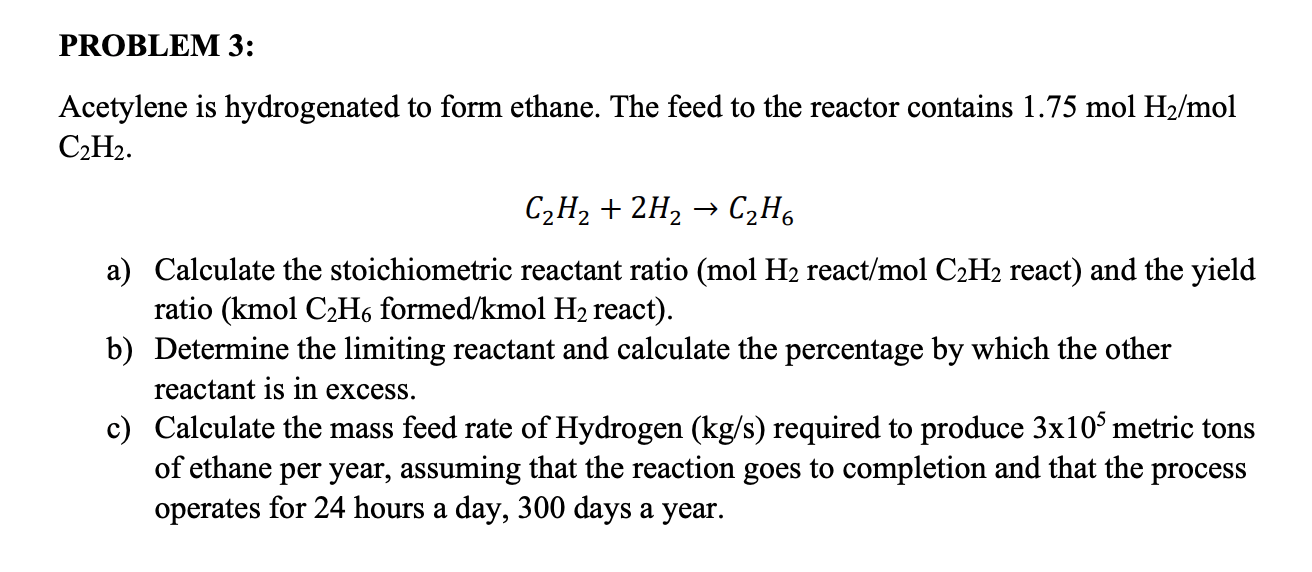
Acetylene Is Hydrogenated To Form Ethane
Acetylene Is Hydrogenated To Form Ethane - Acetylene (c2h2) is hydrogenated to form ethane (c2h6). We know that the reaction is \(c_2h_2 + 2h_2 → c_2h_6\). Q.3 acetylene is hydrogenated to form ethane in the following reaction c₂h₂ + 2h₂ → c₂h6. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react). The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: The feed to the reactor contains 1.50 mol h2/mol c2h2. The balanced chemical equation for this. C2h2 + 2 h2 → c2h6. This tells us that for every mole of acetylene, we need two moles of hydrogen gas, and we form 1 mole of ethane. Acetylene (c2h2) is hydrogenated to form ethane (c2h). The feed to the reactor contains 1.50 mol h2/mol c2h2. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2. The balanced chemical equation for this. The feed to the reactor contains 1.50mol h2/mol c2h2. The feed to the reactor contains 1.50 mol h2/mol c2h2. Acetylene is hydrogenated to form ethane (c2h6). The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: From this equation, we can see that 1 mole of c2h2 reacts with 2. Question #2 (15 marks) acetylene (c2h2) is hydrogenated to form ethane (c2h6). Acetylene is hydrogenated to form ethane. C2h2 + 2 h2 → c2h6. From the stoichiometry, 2 moles of h2 react. A gas containing methane, ethane, and carbon dioxide is analyzed with a gas chromatograph (gc) and flame ionization detector (fid): (a) calculate the stoichiometric reactant ratio (mol. The feed to the reactor contains 1.50 mol h2/mol c2h2. From this equation, we can see that 1 mole of c2h2 reacts with 2. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react). (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2. Calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react) and the. The feed to the reactor contains 1.50 mol h_2/mol c_2h_2. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react). Q.3 acetylene is hydrogenated to form ethane in the following reaction c₂h₂ + 2h₂ → c₂h6. Acetylene is hydrogenated to form ethane in the following reaction: From this equation, we can see that 1 mole of c2h2 reacts with 2. Acetylene is hydrogenated to form ethane (c2h6). Calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react). The gc separates the components of the gas, and. The feed to thereactor contains 1.5 mol h2/mol c2h2.a. Acetylene is hydrogenated to form ethane. Calculate the stoichiometric reactant ratio (mole h_2 react/mol c_2h_2 react) and. We know that the reaction is \(c_2h_2 + 2h_2 → c_2h_6\). Calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react). When acetylene is hydrogenated with molecular hydrogen, two molecules of hydrogen adds up to the acetylene and convert it into ethane. From this equation, we can see that 1 mole of c2h2 reacts with 2. The feed to the. The feed to thereactor contains 1.5 mol h2/mol c2h2.a. Acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h_2/mol c_2h_2. The gc separates the components of the gas, and. The reaction between acetylene (c2h2) and hydrogen (h2) to form ethane (c2h6) can be represented as: C 2 h 2 + 2 h 2 → c 2 h 6 the feed to the reactor contains 1.25 mol h2/mol c2h2. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2. Question #2 (15 marks) acetylene (c2h2) is hydrogenated to form ethane (c2h6). Acetylene is hydrogenated to form ethane. The feed to the reactor contains 1.50 mol h_2/mol. Acetylene (c2h2) is hydrogenated to form ethane (c2h6). The feed to the reactor contains 1.50 mol h2/mol c2h2. (a) the balanced equation for the hydrogenation of acetylene (c2h2) to form ethane (c2h6) is: Acetylene (c2h2) is hydrogenated to form ethane (c2h). A gas containing methane, ethane, and carbon dioxide is analyzed with a gas chromatograph (gc) and flame ionization detector. The feed to the reactor contains 1.25 mol h₂/mol c₂h₂. The feed to the reactor contains 1.50 mol h2/mol ch2. From the stoichiometry, 2 moles of h2 react. This tells us that for every mole of acetylene, we need two moles of hydrogen gas, and we form 1 mole of ethane. Acetylene (c2h2) is hydrogenated to form ethane (c2h6). Acetylene (c2h2) is hydrogenated to form ethane (c2h6). Q.3 acetylene is hydrogenated to form ethane in the following reaction c₂h₂ + 2h₂ → c₂h6. Calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react). Acetylene (c2h2) is hydrogenated to form ethane (c2h). C 2 h 2 + 2 h 2 → c 2 h 6 the feed to the reactor. Draw and label the flowchart. The feed to thereactor contains 1.5 mol h2/mol c2h2.a. This tells us that for every mole of acetylene, we need two moles of hydrogen gas, and we form 1 mole of ethane. Calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react) and the. Acetylene (c2h2) is hydrogenated to form ethane (c2h6). C2h2 + 2 h2 → c2h6. The feed to the reactor contains 1.50 mol h2/mol c2h2. The feed to the reactor contains 1.50 mol hp/mol c2h. The gc separates the components of the gas, and. Calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react). Acetylene is hydrogenated to form ethane. We know that the reaction is \(c_2h_2 + 2h_2 → c_2h_6\). The feed to the reactor contains 1.50 mol h2/mol ch2. The feed to the reactor contains 1.50 mol h_2/mol c_2h_2. Acetylene is hydrogenated to form ethane in the following reaction: Calculate the stoichiometric reactant ratio (mole h_2 react/mol c_2h_2 react) and.Solved 2. Acetylene is hydrogenated to form ethane. The feed
SOLVED Acetylene reacts with hydrogen in the presence of a catalyst to
Solved 2. Acetylene is hydrogenated to form ethane. The
Solved by an EXPERT Acetylene is hydrogenated to form ethane in the
Solved Hydrogenation of acetylene to form ethane is carried
Solved Q.3 Acetylene is hydrogenated to form ethane in the
Solved PROBLEM 3 Acetylene is hydrogenated to form ethane.
SOLVED Acetylene is hydrogenated to form ethane. The feed to the
Solved Acetylene (C2H2) is hydrogenated to form ethane
Solved 4. a) The hydrogenation of acetylene to form ethane
C 2 H 2 + 2 H 2 → C 2 H 6 The Feed To The Reactor Contains 1.25 Mol H2/Mol C2H2.
A Gas Containing Methane, Ethane, And Carbon Dioxide Is Analyzed With A Gas Chromatograph (Gc) And Flame Ionization Detector (Fid):
(A) The Balanced Equation For The Hydrogenation Of Acetylene (C2H2) To Form Ethane (C2H6) Is:
Calculate The Stoichiometric Reactant Ratio (Mol H2 React/Molc2H2 React).
Related Post: