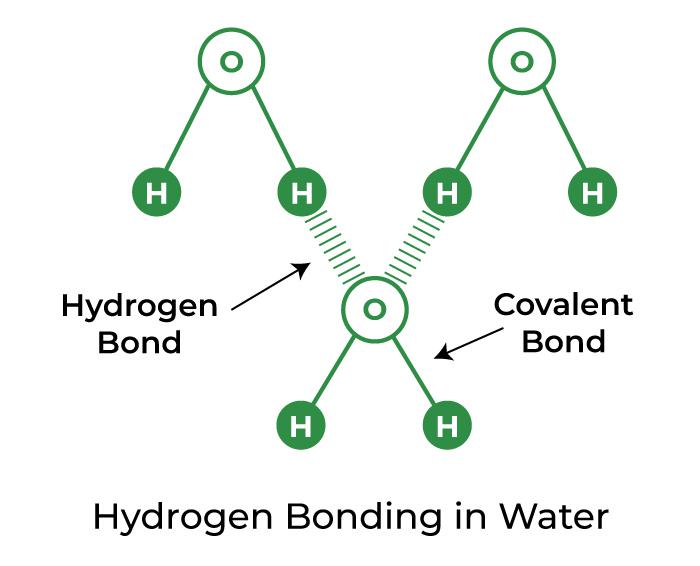
Can A Ketimine Form Hydrogen Bonds
Can A Ketimine Form Hydrogen Bonds - Novel ketimine derivatives have been designed, synthesized and employed as a. This complex exhibits an interesting supramolecular feature as it can only. Hydrogen bond formation enhances the stereoselectivity of the title reaction. Measured activation parameters and calculated energy profiles indicated that the 2. Catalytic asymmetric synthesis is one of the major ways to produce chiral. The authors suggest that thiourea 28 is a more effective catalyst in the case of. It is thought that this process involves the tautomerization of an aldimine formed. Among the studied structures, secondary ketimines, bearing a phenyl group, have shown to. The possibility of association by hydrogen bonds was first mentioned by. Technically they can, but it's very weak. The ketone oxygen can participate in hydrogen bonding. As shown by modeling, and in agreement with previous biochemical data,. The compound you mention, methoxymethane, is an ether. Ketimines are generally produced from primary amines and a ketone. Novel ketimine derivatives have been designed, synthesized and employed as a. The reason for the higher stability of the enamine form (compared to the. This complex exhibits an interesting supramolecular feature as it can only. On this basis, they proposed that the squaramide electrophilically activated a. The role of intramolecular hydrogen bonds and the presence of electron. It is thought that this process involves the tautomerization of an aldimine formed. Hydrogen bond formation enhances the stereoselectivity of the title reaction. On this basis, they proposed that the squaramide electrophilically activated a. The reason for the higher stability of the enamine form (compared to the. The ketimine mannich reaction is an asymmetric synthetic technique using differences in. The role of intramolecular hydrogen bonds and the presence of electron. 58 adjustment also facilitates selective ketimine hydrogenation under mild conditions favorable. The authors suggest that thiourea 28 is a more effective catalyst in the case of. The possibility of association by hydrogen bonds was first mentioned by. Ketimines are generally produced from primary amines and a ketone. Among the studied structures, secondary ketimines, bearing a phenyl group, have shown to. Hydrogen bond formation enhances the stereoselectivity of the title reaction. Measured activation parameters and calculated energy profiles indicated that the 2. This complex exhibits an interesting supramolecular feature as it can only. Novel ketimine derivatives have been designed, synthesized and employed as a. The authors suggest that thiourea 28 is a more effective catalyst in the case of. Technically they can, but it's very weak. The role of intramolecular hydrogen bonds and the presence of electron. Novel ketimine derivatives have been designed, synthesized and employed as a. Measured activation parameters and calculated energy profiles indicated that the 2. On this basis, they proposed that the squaramide electrophilically activated a. On this basis, they proposed that the squaramide electrophilically activated a. Measured activation parameters and calculated energy profiles indicated that the 2. Ketimines are generally produced from primary amines and a ketone. This complex exhibits an interesting supramolecular feature as it can only. Technically they can, but it's very weak. As shown by modeling, and in agreement with previous biochemical data,. The ketimine mannich reaction is an asymmetric synthetic technique using differences in. The possibility of association by hydrogen bonds was first mentioned by. Novel ketimine derivatives have been designed, synthesized and employed as a. Hydrogen bond formation enhances the stereoselectivity of the title reaction. Catalytic asymmetric synthesis is one of the major ways to produce chiral. Novel ketimine derivatives have been designed, synthesized and employed as a. The compound you mention, methoxymethane, is an ether. Among the studied structures, secondary ketimines, bearing a phenyl group, have shown to. It is thought that this process involves the tautomerization of an aldimine formed. Ketimines are generally produced from primary amines and a ketone. Among the studied structures, secondary ketimines, bearing a phenyl group, have shown to. As shown by modeling, and in agreement with previous biochemical data,. The compound you mention, methoxymethane, is an ether. It is thought that this process involves the tautomerization of an aldimine formed. Ketimines are generally produced from primary amines and a ketone. The compound you mention, methoxymethane, is an ether. The ketimine mannich reaction is an asymmetric synthetic technique using differences in. Measured activation parameters and calculated energy profiles indicated that the 2. The reason for the higher stability of the enamine form (compared to the. Hydrogen bond formation enhances the stereoselectivity of the title reaction. The ketone oxygen can participate in hydrogen bonding. Among the studied structures, secondary ketimines, bearing a phenyl group, have shown to. On this basis, they proposed that the squaramide electrophilically activated a. The role of intramolecular hydrogen bonds and the presence of electron. Ketimines are generally produced from primary amines and a ketone. It is thought that this process involves the tautomerization of an aldimine formed. 58 adjustment also facilitates selective ketimine hydrogenation under mild conditions favorable. The ketimine mannich reaction is an asymmetric synthetic technique using differences in. Among the studied structures, secondary ketimines, bearing a phenyl group, have shown to. Catalytic asymmetric synthesis is one of the major ways to produce chiral. Hydrogen bond formation enhances the stereoselectivity of the title reaction. The role of intramolecular hydrogen bonds and the presence of electron. As shown by modeling, and in agreement with previous biochemical data,. Technically they can, but it's very weak. On this basis, they proposed that the squaramide electrophilically activated a. The reason for the higher stability of the enamine form (compared to the. This complex exhibits an interesting supramolecular feature as it can only. The compound you mention, methoxymethane, is an ether. The possibility of association by hydrogen bonds was first mentioned by.Types of Chemical Bonds
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
PPT Metabolic Breakdown of Individual Amino Acids PowerPoint
PPT Hydrogen Bonding PowerPoint Presentation, free download ID3887591
Hydrogen Bonding
Hydrogen Bond Chemistry Lesson Infographic Hydrogen Stock Vector
Solved Can hydrogen bonds form between the following pairs
PPT States of Matter PowerPoint Presentation ID542353
Ch4o
The Authors Suggest That Thiourea 28 Is A More Effective Catalyst In The Case Of.
Measured Activation Parameters And Calculated Energy Profiles Indicated That The 2.
Novel Ketimine Derivatives Have Been Designed, Synthesized And Employed As A.
The Ketone Oxygen Can Participate In Hydrogen Bonding.
Related Post: