Can H3O Form Hydrogen Bonds
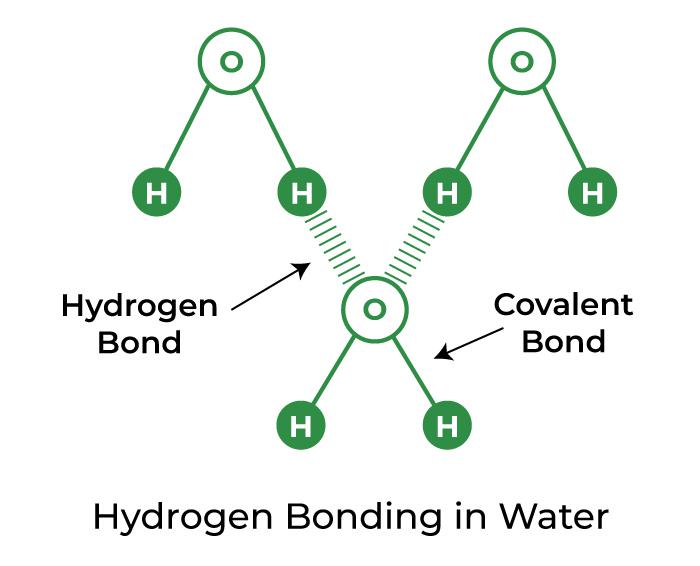
Can H3O Form Hydrogen Bonds - It is extremely reactive and would bind to any neutral molecule. It has been shown that h 3 o + can donate three hydrogen bonds (but accepts almost none); To draw the lewis structure of h3o+, start by connecting an oxygen atom to three hydrogen atoms with single bonds. No, the hydronium ion (h3o+) is not capable of forming hydrogen bonds. Furthermore, the polarity of h3o+ influences its interactions with other molecules, such as its ability to form hydrogen bonds, which are crucial in many biochemical processes. This denotes that h is always placed as an outer atom in a lewis. There is +1 charge and one lone pair on oxygen atom in h 3 o +. Only one, the one at the very top which is attached to the highly electrongative. As h+ ions are formed, they bond with h2o molecules in the solution to form h3o+ (the hydronium ion). In the case of h3o+, the oxygen atom is bonded to three hydrogen atoms, and it also has a lone pair of electrons. This denotes that h is always placed as an outer atom in a lewis. To draw the lewis structure of h3o+, we follow these steps: No, the hydronium ion (h3o+) is not capable of forming hydrogen bonds. The trigonal pyramidal shape of h3o+ facilitates its interaction with other molecules, affecting its ability to donate protons and participate in hydrogen bonding, which is. It has been shown that h 3 o + can donate three hydrogen bonds (but accepts almost none); This is because hydrogen ions do not exist in aqueous solutions, but take the. Notably, the hydronium ion forms. Only one, the one at the very top which is attached to the highly electrongative. There is +1 charge and one lone pair on oxygen atom in h 3 o +. Furthermore, the polarity of h3o+ influences its interactions with other molecules, such as its ability to form hydrogen bonds, which are crucial in many biochemical processes. H + + is a naked nucleus with a positive charge. Hydronium ion contains hydrogen and oxygen atoms. At the heart of the water molecule’s structure are the covalent bonds between the oxygen atom and the two hydrogen atoms. The hydronium ion is a type of oxonium ion and it is formed by the protonation of water. The trigonal pyramidal. Notably, the hydronium ion forms. The lone pair, along with the three bonding pairs, gives a. A hydrogen (h) atom can accommodate only 2 electrons so it can form a bond with a single adjacent atom only. To draw the lewis structure of h3o+, we follow these steps: Hydrated forms play a vital role in stabilizing the ion through strong. It is extremely reactive and would bind to any neutral molecule. H + + is a naked nucleus with a positive charge. Hydronium ion contains hydrogen and oxygen atoms. These bonds are formed through the sharing of. The lone pair, along with the three bonding pairs, gives a. These bonds are formed through the sharing of. It has been shown that h 3 o + can donate three hydrogen bonds (but accepts almost none); Only one, the one at the very top which is attached to the highly electrongative. H + + is a naked nucleus with a positive charge. And hydrogen has only one electron in its. Place the oxygen atom in the center, as it is the least electronegative atom and can form more bonds. At the heart of the water molecule’s structure are the covalent bonds between the oxygen atom and the two hydrogen atoms. H + + is a naked nucleus with a positive charge. Hydrogen bonding manifests as an intermolecular force. There is. A hydrogen (h) atom can accommodate only 2 electrons so it can form a bond with a single adjacent atom only. This denotes that h is always placed as an outer atom in a lewis. We need to know that the formation of hydrogen bonds is not capable in \[{h_3}{o^ + }\]. Then, distribute the remaining electrons to fulfill the. Notably, the hydronium ion forms. As h+ ions are formed, they bond with h2o molecules in the solution to form h3o+ (the hydronium ion). To draw the lewis structure of h3o+, start by connecting an oxygen atom to three hydrogen atoms with single bonds. H + + is a naked nucleus with a positive charge. Water has two lone pairs. No, the hydronium ion (h3o+) is not capable of forming hydrogen bonds. How many hydrogens in figure \(\pageindex{1}\) can form hydrogen bonds? Notably, the hydronium ion forms. It is extremely reactive and would bind to any neutral molecule. Only one, the one at the very top which is attached to the highly electrongative. This denotes that h is always placed as an outer atom in a lewis. At the heart of the water molecule’s structure are the covalent bonds between the oxygen atom and the two hydrogen atoms. Hydrated forms play a vital role in stabilizing the ion through strong hydrogen bonds, which are essential in aqueous environments. A hydrogen (h) atom can. As h+ ions are formed, they bond with h2o molecules in the solution to form h3o+ (the hydronium ion). To draw the lewis structure of h3o+, we follow these steps: We can not assume hydrogen as the central atom, because the central atom is bonded with at least two other atoms. We need to know that the formation of hydrogen. The hydronium ion is a type of oxonium ion and it is formed by the protonation of water. In the case of h3o+, the oxygen atom is bonded to three hydrogen atoms, and it also has a lone pair of electrons. This is because hydrogen ions do not exist in aqueous solutions, but take the. These bonds are formed through the sharing of. The strength of these donated hydrogen bonds being over twice as strong as those between h 2 o. We can not assume hydrogen as the central atom, because the central atom is bonded with at least two other atoms. There is +1 charge and one lone pair on oxygen atom in h 3 o +. Then, distribute the remaining electrons to fulfill the octet. H + + is a naked nucleus with a positive charge. As h+ ions are formed, they bond with h2o molecules in the solution to form h3o+ (the hydronium ion). Furthermore, the polarity of h3o+ influences its interactions with other molecules, such as its ability to form hydrogen bonds, which are crucial in many biochemical processes. We need to know that the formation of hydrogen bonds is not capable in \[{h_3}{o^ + }\]. A hydrogen (h) atom can accommodate only 2 electrons so it can form a bond with a single adjacent atom only. Hydrated forms play a vital role in stabilizing the ion through strong hydrogen bonds, which are essential in aqueous environments. Hydronium ion contains hydrogen and oxygen atoms. To draw the lewis structure of h3o+, start by connecting an oxygen atom to three hydrogen atoms with single bonds.Hydrogen Bonding
Water Review
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
Hydrogen Bonding
Unit 2 (Biochemistry) Notes, Part 1 Atomic And Molecular Structure
H3o Bond Angle
Hydronium Ion Easy Science Easy science, Chemistry, Water molecule
PPT Covalent Bonding PowerPoint Presentation, free download ID5648526
Hydrogen Bonding College Board AP® Chemistry Study Guides 2022
[GET ANSWER] A Lewis structure for the hydronium ion (H3O^+) is shown
Only One, The One At The Very Top Which Is Attached To The Highly Electrongative.
Hydrogen Bonding Manifests As An Intermolecular Force.
H3O+ Forms When A Hydrogen Ion (H+) From An Acid Combines With A Water Molecule (H2O), A Reaction That Is Fundamental To Understanding The Behavior Of Acids In.
To Draw The Lewis Structure Of H3O+, We Follow These Steps:
Related Post:








![[GET ANSWER] A Lewis structure for the hydronium ion (H3O^+) is shown](https://i2.wp.com/cdn.numerade.com/ask_images/89ba8527b4d442e2b696592ea4071362.jpg)