How Many Atoms Are Needed To Form A Molecule
How Many Atoms Are Needed To Form A Molecule - This is because a molecule is a group of atoms bonded together, and the simplest molecule consists of two atoms bonded to each other And if we combine two or more, then molecules are formed under. It seemed clear from the context that they were including (and in fact referring. Molecules can be made up of the same type of atoms (e.g., o2, n2) or different types of atoms (e.g., h2o, co2) 3 determine the minimum number of atoms required. A molecule is formed when two or more atoms bond together. A molecule is formed when two or more. To form 5 water molecules, you need 10 hydrogen atoms, as each water molecule comprises 2 hydrogen atoms. A mole is defined as 6.02 × 1023 items and thus, it is used by chemists to represent a large number of atoms or molecules. Therefore, the calculation is 5 molecules times 2 hydrogen. Here’s the best way to solve it. We need a number that represents billions and billions of atoms. A molecule is two or more atoms connected by chemical bonds, which form the smallest unit of a substance that retains the composition and properties of that substance. The number of atoms required to form a molecule depends on the specific molecule. The simplest molecule is made up of just two atoms, like oxygen gas (o 2) or. Molecules can be small, like water molecules, or they can be large, like proteins. In order to create molecules, atoms must combine. So, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules. Because 1 n 2 molecule contains 2 n atoms, 1 mol of n 2 molecules (6.022 × 10 23 molecules) has 2 mol of n atoms. Because 1 n 2 molecule contains 2 n atoms, 1 mol of n 2 molecules (6.022 × 10 23 molecules) has 2 mol of n atoms. To form 5 water molecules, you need 10 hydrogen atoms, as each water molecule comprises 2 hydrogen atoms. A molecule is formed when two or more atoms bond together. Post any question and get expert help quickly. Some molecules are composed of just two atoms. It seemed clear from the context that they were including (and in fact referring. Chemists use the term mole to represent a large number of atoms or molecules. Therefore, the calculation is 5 molecules times 2 hydrogen. Therefore, you need 2 oxygen atoms to form one molecule of oxygen. The minimum number of atoms required to form a molecule is two. So, minimum 2 atoms are required to form a molecule. Molecules can be made up of the same type of atoms (e.g., o2, n2) or different types. For example, oxygen gas (o₂). Molecules can be small, like water molecules, or they can be large, like proteins. A molecule is formed when two or more. Here’s the best way to solve it. Molecular oxygen, represented as o2, consists of two oxygen atoms connected by a double covalent bond. Different atoms combine together to form. It seemed clear from the context that they were including (and in fact referring. Molecules can be made up of the same type of atoms (e.g., o2, n2) or different types of atoms (e.g., h2o, co2) 3 determine the minimum number of atoms required. Molecules can be small, like water molecules, or they can. To form a molecule, at least two atoms are required. How many atoms are required to form a molecule? Group of two or more atoms held together by chemical bonds. So, minimum 2 atoms are required to form a molecule. A mole is defined as 6.02 × 1023 items and thus, it is used by chemists to represent a large. So, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules. Here’s the best way to solve it. Using formulas to indicate how many atoms of each element we have in. How many atoms are required to form a molecule? Group of two or more atoms held. How many atoms are required to form a molecule? Just as a dozen implies 12 things, a mole. Different atoms combine together to form. For example, oxygen gas (o₂). Group of two or more atoms held together by chemical bonds. The number of atoms required to form a molecule depends on the specific molecule. This is because a molecule is a group of atoms bonded together, and the simplest molecule consists of two atoms bonded to each other In order to create molecules, atoms must combine. It seemed clear from the context that they were including (and in fact referring.. Different atoms combine together to form. Here’s the best way to solve it. Using formulas to indicate how many atoms of each. Because 1 n 2 molecule contains 2 n atoms, 1 mol of n 2 molecules (6.022 × 10 23 molecules) has 2 mol of n atoms. Therefore, you need 2 oxygen atoms to form one molecule of oxygen. Some molecules are composed of just two atoms. Using formulas to indicate how many atoms of each element we have in. I recently saw a quote from an expert which said it takes three atoms to form a molecule. When two atoms are joined, molecules are also formed in this situation. Just as a dozen implies 12 things, a mole. To form 5 water molecules, you need 10 hydrogen atoms, as each water molecule comprises 2 hydrogen atoms. A mole is defined as 6.02 × 1023 items and thus, it is used by chemists to represent a large number of atoms or molecules. This is because a molecule is a group of atoms bonded together, and the simplest molecule consists of two atoms bonded to each other Post any question and get expert help quickly. And the number of atoms which are utilized for the. Covalent molecules can range from 2 atoms (e.g., h2) to thousands of atoms (e.g.,. Because 1 n 2 molecule contains 2 n atoms, 1 mol of n 2 molecules (6.022 × 10 23 molecules) has 2 mol of n atoms. Molecular oxygen, represented as o2, consists of two oxygen atoms connected by a double covalent bond. A molecule is formed when two or more atoms bond together. Here’s the best way to solve it. To form a molecule, at least two atoms are required. A molecule is formed when two or more. The number of atoms required to form a molecule depends on the specific molecule. We need a number that represents billions and billions of atoms. Just as a dozen implies 12 things, a mole. So, from the above two facts it is clear that, minimum two atoms or maximum infinite atoms are required for the formation of molecules.PPT Chapter 9 Chemical Reactions PowerPoint Presentation, free
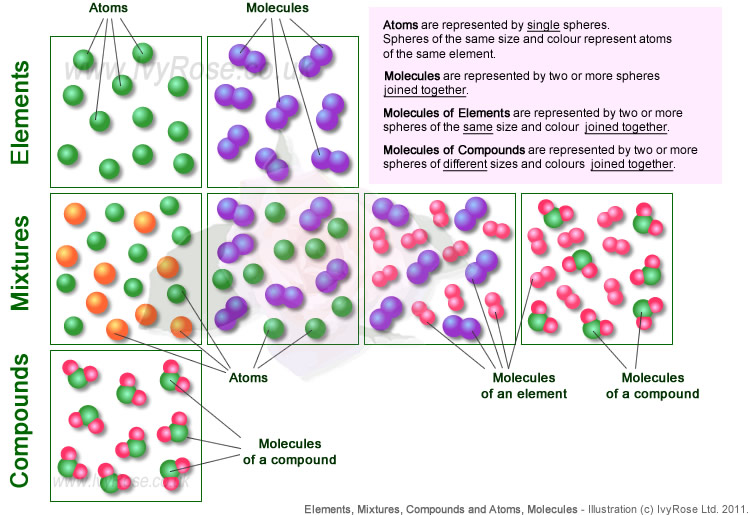
Elements, Mixtures and Compounds vs Atoms and Molecules School Chemistry
Chapter 6 Quantities in Chemical Reactions Chemistry
Describe the formation of nitrogen molecule.
LabXchange
How Do Atoms Form Covalent Bond vrogue.co
Vocabulary. Vocabulary Chemical Equations Balancing Act. ppt download
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
PPT Chemistry Introduction to Matter PowerPoint Presentation, free
Solved How Many Atoms In The Pictured Molecule Can Form H...
Because 1 N 2 Molecule Contains 2 N Atoms, 1 Mol Of N 2 Molecules (6.022 × 10 23 Molecules) Has 2 Mol Of N Atoms.
Using Formulas To Indicate How Many Atoms Of Each Element We Have In.
Using Formulas To Indicate How Many Atoms Of Each.
Molecules Can Be Made Up Of The Same Type Of Atoms (E.g., O2, N2) Or Different Types Of Atoms (E.g., H2O, Co2) 3 Determine The Minimum Number Of Atoms Required.
Related Post: