How Many Hydrogen Bonds Can A Single Water Molecule Form
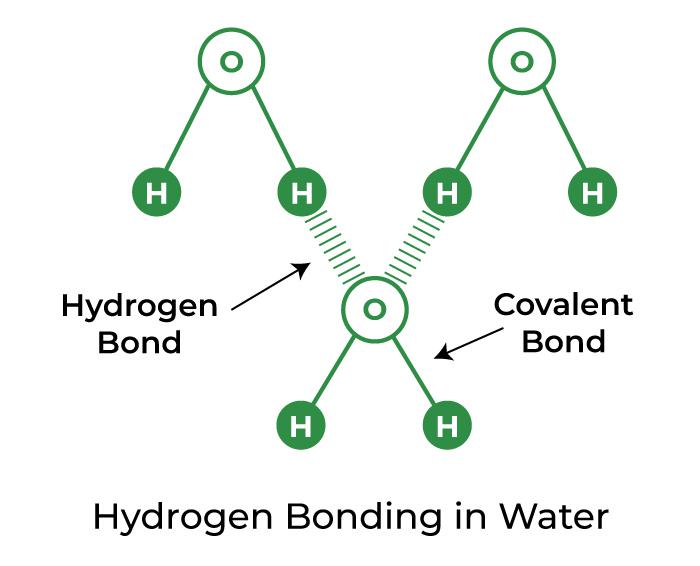
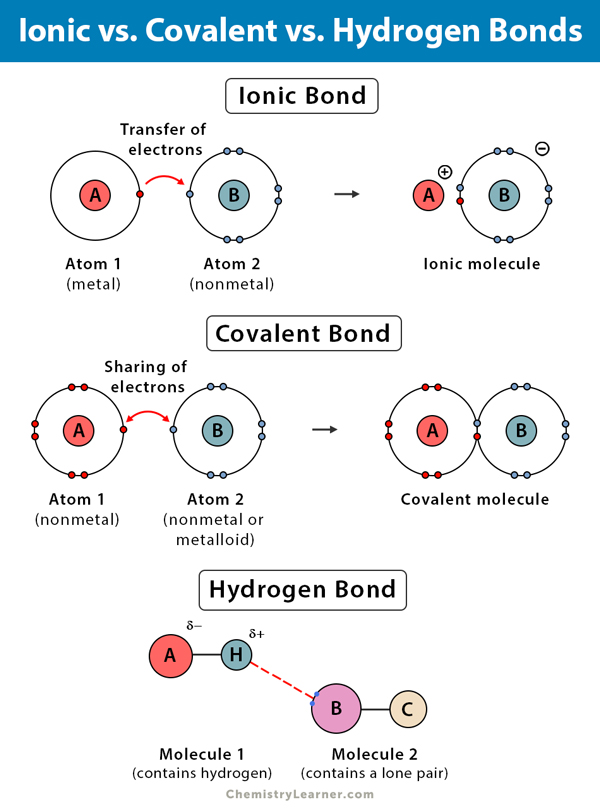
How Many Hydrogen Bonds Can A Single Water Molecule Form - The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. During the combustion of methane (ch4) to form water (h2o) and carbon dioxide (co2), the bonds broken are the. Water can form four bonds. The bond in a hydrogen molecule, measured as the distance between the two. Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule. How many hydrogen bonds can a single water molecule form? Positive hydrogen of one molecule attracted to negative oxygen of nearby molecule. Hydrogen bonding occurs when the slightly positive hydrogen of one molecule is attracted to the slightly negative oxygen of a. A single water molecule can form up to four hydrogen bonds. Study with quizlet and memorize flashcards containing terms like explain hydrogen bonding. The lone pairs, demanding more space, compress the bond angle between the hydrogen atoms to approximately 104.5 degrees. Each water molecule can form: A single water molecule can form up to four hydrogen bonds with surrounding water molecules. Hydrogen bonding occurs when the slightly positive hydrogen of one molecule is attracted to the slightly negative oxygen of a. Cohesion is the phenomenon of the. Water (h₂o) has two hydrogen atoms and two lone pairs of electrons. A single water molecule can form up to four hydrogen bonds. The sheer volume of information contained in a single nmr spectrum always impressed me, though: You get to see all the hydrogen atoms (well, most all of them), and how. In polar covalent water, positive hydrogen atoms bond to the negative oxygen atom of another water molecule. Water (h₂o) has two hydrogen atoms and two lone pairs of electrons. Water can form four bonds. Each water molecule can form 4 hydrogen bonds. The lone pairs, demanding more space, compress the bond angle between the hydrogen atoms to approximately 104.5 degrees. How many hydrogen bonds can a single water molecule form? How many hydrogen bonds can a single water molecule form?, cohesion, adhesion and more. This happens because each water molecule has two hydrogen atoms that can. In this question, we need to determine the maximum. Instead, a water molecule can form and. A single water molecule can form up to four hydrogen bonds. Water (h₂o) has two hydrogen atoms and two lone pairs of electrons. A single water molecule can form up to four hydrogen bonds with surrounding water molecules. A single water molecule can form up to four hydrogen bonds. How many hydrogen bonds can a single water molecule form? Cohesion is the phenomenon of the. Identify four properties of water that are important for life and describe how they result from hydrogen bonding. Each water molecule can form: Water can form four bonds. You get to see all the hydrogen atoms (well, most all of them), and how. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the. Instead, a water molecule can form and. This bent geometry imparts a significant. Water (h₂o) has two hydrogen atoms and two lone pairs of electrons. How many hydrogen bonds can a single water molecule form? How many hydrogen bonds can a single water molecule form?, cohesion, adhesion and more. Remember that the dash, also referred to as a single bond, represents a pair of electrons. You get to see all the hydrogen atoms (well, most all of them), and how. Distinguish between cohesion and adhesion. A single water molecule can form 4 hydrogen bonds. How many hydrogen bonds can a single water molecule form?, cohesion, adhesion and more. A single water molecule can form 4 hydrogen bonds. The sheer volume of information contained in a single nmr spectrum always impressed me, though: Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. The bond in a hydrogen molecule, measured as the distance between the two. A. How many hydrogen bonds can a single water molecule form? In polar covalent water, positive hydrogen atoms bond to the negative oxygen atom of another water molecule. Cohesion is the phenomenon of the. How many hydrogen bonds can a single water molecule form? Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards. This bent geometry imparts a significant. Hydrogen bonding occurs when the slightly positive hydrogen of one molecule is attracted to the slightly negative oxygen of a. The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. Water (h₂o) has two hydrogen atoms and two lone pairs of electrons. A. This bent geometry imparts a significant. Study with quizlet and memorize flashcards containing terms like explain hydrogen bonding. The lone pairs, demanding more space, compress the bond angle between the hydrogen atoms to approximately 104.5 degrees. You get to see all the hydrogen atoms (well, most all of them), and how. Instead, a water molecule can form and. The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. How many hydrogen bonds can a single water molecule form?, cohesion, adhesion and more. The lone pairs, demanding more space, compress the bond angle between the hydrogen atoms to approximately 104.5 degrees. Water can form four bonds. Instead, a water molecule can form and. During the combustion of methane (ch4) to form water (h2o) and carbon dioxide (co2), the bonds broken are the. Hydrogen bonding occurs when the slightly positive hydrogen of one molecule is attracted to the slightly negative oxygen of a. Each water molecule can form: How many hydrogen bonds can a single water molecule form? Remember that the dash, also referred to as a single bond, represents a pair of electrons. A single water molecule can form 4 hydrogen bonds. In polar covalent water, positive hydrogen atoms bond to the negative oxygen atom of another water molecule. Cohesion is the phenomenon of the. A single water molecule can form up to four hydrogen bonds. A single water molecule can form up to four hydrogen bonds. How many hydrogen bonds can a single water molecule form?Water Review
PPT Water Chemistry & Properties of Water PowerPoint Presentation
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
Showing a water molecule with Hydrogen bonds There are still two
Water Videos
how many hydrogen bonds can a single water molecule form
Hydrogen Bonding
Water. ppt download
The diagram shows hydrogen bonds between water molecules. Label the
Water Molecule Hydrogen Bond Diagram Hydrogen Bonds Give Water Unique
Study With Quizlet And Memorize Flashcards Containing Terms Like Explain Hydrogen Bonding.
Distinguish Between Cohesion And Adhesion.
In This Question, We Need To Determine The Maximum.
Hydrogen Bonds Form Between The Hydrogen Atom Of One Water Molecule And The Lone Pair On The Oxygen Atom Of Another Molecule.
Related Post: