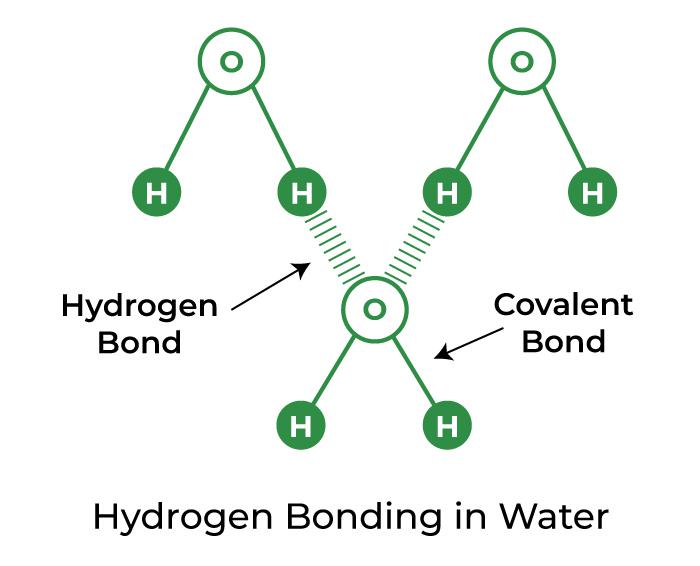
How Many Hydrogen Bonds Can Water Form
How Many Hydrogen Bonds Can Water Form - Scientists have hypothesized since the 1960s that the sun is a source of ingredients that form water on the moon. The formation of a water molecule from two hydrogen atoms and an oxygen atom can be illustrated using lewis dot symbols (shown below). In this question, we need to determine the maximum. The oxygen atom in water has two lone pairs of electrons, each capable of accepting a hydrogen bond. Each hydrogen can only bond. When hydrogen and oxygen combine to form water, a total of two bonds are formed. These four hydrogen bonds arrange themselves. Water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule. A single water molecule (h₂o) can form up to four hydrogen bonds. Hydrogen bonding forms in liquid water as the hydrogen atoms of one water molecule are attracted towards the oxygen atom of a neighboring water molecule. Each water molecule can form up to four hydrogen bonds, because they have two partially positively charged hydrogen atoms and two electron lone pairs. A single water molecule can form up to four hydrogen bonds. Each hydrogen atom can form one hydrogen bond. Transitioning to the liquid phase, water molecules engage in extensive hydrogen bonding, where a single water molecule can form up to four hydrogen bonds—two as a. Each hydrogen can only bond. Since there are two hydrogen atoms in a water molecule, they can each form one hydrogen bond. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. Water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Each hydrogen can only bond. The number of electrons required. Each hydrogen atom can form one hydrogen bond. Scientists have hypothesized since the 1960s that the sun is a source of ingredients that form water on the moon. Since there are two hydrogen atoms in a water molecule, they can each form one hydrogen bond. Each hydrogen atom can form one hydrogen bond. In this question, we need to determine the maximum. The formation of a water molecule from two hydrogen atoms and an oxygen atom can be illustrated using lewis dot symbols (shown below). Each water molecule. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: The oxygen atom in water has two lone pairs of electrons, each capable of accepting a hydrogen bond. When a stream of charged particles known as. Each water molecule can form up to four hydrogen bonds, because they have two partially positively charged hydrogen atoms. Water is an ideal example of hydrogen bonding. The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. When a stream of charged particles known as. Each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds by their oxygen atom. Notice that. A single water molecule (h₂o) can form up to four hydrogen bonds. The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. These four hydrogen bonds arrange themselves. When a stream of charged particles known as. Each hydrogen atom can form one hydrogen bond. Water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. When hydrogen and oxygen combine to form water, a total of two bonds are formed. Transitioning to the liquid phase, water molecules engage in extensive hydrogen bonding, where a single water molecule can form up to four. Water is an ideal example of hydrogen bonding. The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. When hydrogen and oxygen combine to form water, a total of two bonds are formed. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Hydrogen. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: These four hydrogen bonds arrange themselves. Transitioning to the liquid phase, water molecules engage in extensive hydrogen bonding, where a single water molecule can form up to four hydrogen bonds—two as a. The oxygen atom in water has two lone pairs of electrons, each of. When hydrogen and oxygen combine to form water, a total of two bonds are formed. When a stream of charged particles known as. Water is an ideal example of hydrogen bonding. Since there are two hydrogen atoms in a water molecule, they can each form one hydrogen bond. Each hydrogen can only bond. The formation of a water molecule from two hydrogen atoms and an oxygen atom can be illustrated using lewis dot symbols (shown below). In this question, we need to determine the maximum. Each water molecule can form up to four hydrogen bonds, because they have two partially positively charged hydrogen atoms and two electron lone pairs. That means that every. Water is capable of participating in 4 hydrogen bonds at once, granted it only does this when it forms a perfect crystal structure. Hydrogen bonds form between the hydrogen atom of one water molecule and the lone pair on the oxygen atom of another molecule. A single water molecule (h₂o) can form up to four hydrogen bonds. These four hydrogen bonds arrange themselves. Each water molecule can form two hydrogen bonds involving their hydrogen atoms plus two further hydrogen bonds by their oxygen atom. Each hydrogen can only bond. Each water molecule can form up to four hydrogen bonds, because they have two partially positively charged hydrogen atoms and two electron lone pairs. The oxygen atom in water has two lone pairs of electrons, each of which can form a hydrogen bond with a. One bond is formed between each hydrogen atom and the oxygen atom. When hydrogen and oxygen combine to form water, a total of two bonds are formed. The oxygen atom in water has two lone pairs of electrons, each capable of accepting a hydrogen bond. Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: That means that every hydrogen will coordinate. The number of electrons required. Each hydrogen atom can form one hydrogen bond. Transitioning to the liquid phase, water molecules engage in extensive hydrogen bonding, where a single water molecule can form up to four hydrogen bonds—two as a.PPT Lec.1 Chemistry Of Water PowerPoint Presentation, free download
The diagram shows hydrogen bonds between water molecules. Label the
Showing a water molecule with Hydrogen bonds There are still two
Unit 2 (Biochemistry) Notes, Part 1 Atomic And Molecular Structure
Water Videos
Water Review
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
The role of water describe how hydrogen bonding occurs between water
Hydrogen Bonding
How Water Forms Hydrogen Bonds YouTube
The Formation Of A Water Molecule From Two Hydrogen Atoms And An Oxygen Atom Can Be Illustrated Using Lewis Dot Symbols (Shown Below).
Since There Are Two Hydrogen Atoms In A Water Molecule, They Can Each Form One Hydrogen Bond.
When A Stream Of Charged Particles Known As.
In This Question, We Need To Determine The Maximum.
Related Post: