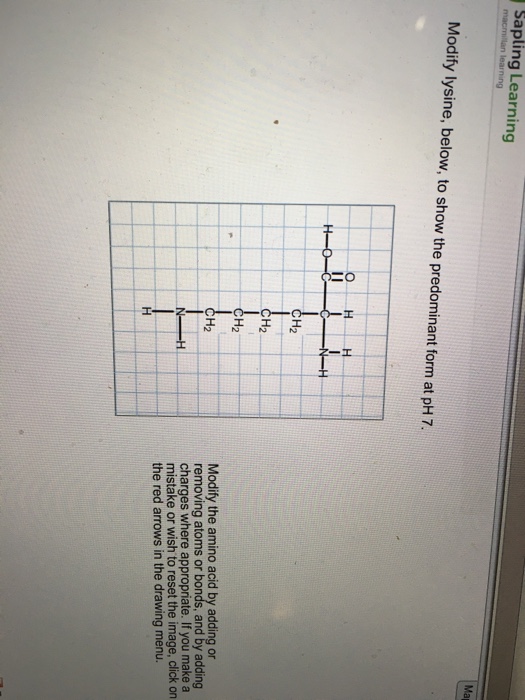
Modify Lysine To Show The Predominant Form At Ph 7
Modify Lysine To Show The Predominant Form At Ph 7 - Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Consider the pka values of the functional groups in lysine and compare them to the given ph value of 7 to determine whether each group should be protonated or deprotonated. This results in a zwitterionic form where the amino. Modify the amino acid by adding or removing atoms or bonds and by. In this form, the amino groups are protonated and the carboxylic acid group is deprotonated. Modify the amino acid by adding or removing atoms or bonds, and by adding charges where. Modify the amino acid by adding charges where appropriate, if you make a mistake or wish to reset the image, click on the red. Lysine has three functional groups: Modify lysine to show the predominant form at ph 7. Your solution’s ready to go! Modify the amino acid by adding or removing atoms or bonds and by. Modify the amino acid by adding charges where appropriate, if you make a mistake or wish to reset the image, click on the red. Modify lysine, below, to show the predominant form at ph 7. Modify lysine to show the predominant form at ph 7. Ch3 ch2 modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. If you make a mistake or wish to reset the image, click on the red arrows in. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. At ph 7, the predominant form of lysine is the zwitterionic form. Modify lysine to show the predominant form at ph7. Modify the amino acid by adding or removing atoms or bonds, and by adding charges where. Lysine has three functional groups: Modify the amino acid by adding or removing atoms or bonds and by. At ph 7, the predominant form of lysine is the zwitterionic form. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify the amino acid by adding or removing atoms or bonds, and by. Answer of modify lysine, below, to show the predominant form at ph 7. This results in a zwitterionic form where the amino. Lysine has three functional groups: To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. To determine the predominant form of lysine at ph. Modify lysine to show the predominant form at ph7. Your solution’s ready to go! Modify the amino acid by adding or removing atoms or bonds, and by adding charges where. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. At ph 7, the predominant form. Consider the pka values of the functional groups in lysine and compare them to the given ph value of 7 to determine whether each group should be protonated or deprotonated. Modify lysine to show the predominant form at ph 7. Modify lysine, below, to show the predominant form at ph 7. Modify the amino acid by adding or removing atoms. Answer of modify lysine, below, to show the predominant form at ph 7. Consider the pka values of the functional groups in lysine and compare them to the given ph value of 7 to determine whether each group should be protonated or deprotonated. Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate.. In this form, the amino groups are protonated and the carboxylic acid group is deprotonated. Consider the pka values of the functional groups in lysine and compare them to the given ph value of 7 to determine whether each group should be protonated or deprotonated. Answer of modify lysine, below, to show the predominant form at ph 7. Modify lysine. Your solution’s ready to go! Modify the amino acid by adding or removing atoms or bonds, and by adding charges where. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. To determine the predominant form of lysine at ph 7, we need to consider the. Modify lysine to show the predominant form at ph 7. Lysine has three functional groups: Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Ch3 ch2 modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. If you make a mistake or wish to reset. Ch3 ch2 modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify lysine to show the predominant form at ph 7. In this form, the amino groups are protonated and the carboxylic acid group is deprotonated. Modify the amino acid by adding or removing atoms or bonds and by adding charges where. Modify the amino acid by adding or removing atoms or bonds, and by adding charges where. Consider the pka values of the functional groups in lysine and compare them to the given ph value of 7 to determine whether each group should be protonated or deprotonated. Modify lysine, below, to show the predominant form at ph 7. At ph 7,. At ph 7, the predominant form of lysine is the zwitterionic form. In this form, the amino groups are protonated and the carboxylic acid group is deprotonated. Modify the amino acid by adding charges where appropriate, if you make a mistake or wish to reset the image, click on the red. Lysine has three functional groups: Modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify lysine to show the predominant form at ph7. If you make a mistake or wish to reset the image, click on the red arrows in. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. This results in a zwitterionic form where the amino. Consider the pka values of the functional groups in lysine and compare them to the given ph value of 7 to determine whether each group should be protonated or deprotonated. To determine the predominant form of lysine at ph 7, we need to consider the pka values of the functional groups present in lysine. Lysine has three functional groups: Modify lysine to show the predominant form at ph 7. Ch3 ch2 modify the amino acid by adding or removing atoms or bonds and by adding charges where appropriate. Modify the amino acid by adding or removing atoms or bonds, and by adding charges where.Modify lysine, below, to show the predominant form at pH 7. Quizlet
Lysine At Ph 7
Modify lysine, below, to show the predominant form at pH 7. Quizlet
Solved Modify lysine to show the predominant form at pH7.
Lysine At Ph 7
Solved Modify lysine, below, to show the predominant form at
Solved modify lysine to show the predominant form at pH 7
Solved Modify lysine to show the predominant form at pH 7.
Solved Modify lysine to show the predominant form at pH 7.
SOLVED Resources Hint Check Modify lysine to show the predominant form
Modify The Amino Acid By Adding Or Removing Atoms Or Bonds And By.
Answer Of Modify Lysine, Below, To Show The Predominant Form At Ph 7.
Your Solution’s Ready To Go!
Modify Lysine, Below, To Show The Predominant Form At Ph 7.
Related Post: